Over the past few years, there has been a major increase in the use of air injection systems that are designed to increase the oxygen content in nutrient solutions for irrigation. It seems that every grower has or is considering the use of these systems in their operations, but what advantages do they have? What are the arguments for and against using these systems, what conditions should they be used under, and what actually happens to the irrigation solution when these systems are used?
The basic chemistry of water

Regular water contains not only water molecules (H2O) but also dissolved solids that can range from substances that are good for plants, like calcium and iron, to those which are not so good, like sodium and lead. Water also contains dissolved gases such as oxygen and carbon dioxide. The amount of dissolved gases held is affected by temperature – the warmer the water is, the less of these gases it will retain – and also by the concentration of dissolved solids – the more dissolved solids there are, the less gases will be held.
When water is moving or being stirred so that it comes into contact with air, the amount of dissolved gases will remain at fairly stable levels. However, when water is left standing still, these gases begin to leave the water, by rising up through the column of water so that there is a lack of dissolved air at the lower levels but levels increase towards the top. This process is known as stagnation and it is the main reason that growers might want to aerate their irrigation water.
Forming of carbonates
While these gases are present in the water, they can affect many things including the physical and ionic states of the elements present in the water and the pH of the water. As carbon dioxide (CO2) moves through the water column, it reacts with ions such as calcium and begins to raise the pH as carbonates are formed.
Most public drinking water supplies take advantage of this mechanism by adding calcium carbonate to the water to serve as a pH buffer that both provides better-tasting water and protects pipes from extreme pH levels – both high and low. The buffer offsets pH changes from either acid-forming compounds or alkaline-forming compounds that might be present in the water, ensuring that the pH remains constant while the water is stored and delivered to the consumer.
Additional reactions can also occur with other gases in the water including oxygen. Oxygen is an oxidizer and as such, will combine with ions in the water to form new compounds. These new compounds will likely come out of solution or become unavailable for plant usage. This is very important when these gases are present in the ion-rich irrigation water that is used to fertilise plants.

Why dissolved air is important
The atmosphere is made up of many different gases and some of these will dissolve into water. Air dissolved in water is important because it can both sustain and inhibit life. In this situation, the important gases are oxygen, in the diatomic form O2, and carbon dioxide, CO2.
Oxygen must be in its diatomic (O2) form to be useful for life. Oxygen in the form of O2-, the reactive form also known as free radicals, is detrimental to all carbon-based life forms because it is looking for something to combine with and carbon is the optimal partner. Oxygen as O2 is the oxygen source for aquatic life, both plants and animals. Peroxide compounds do not work in the same way because the oxygen released is the reactive free radical (H2O2 converts into H2O + O2-). Carbon dioxide is, of course, not required by the root system but it is needed to influence pH by slowing down these fluctuations.
Without oxygen, anaerobic life forms will start to grow and these can be the causal agents for stagnation and the associated smells, as well as the toxins that can be released and a plethora of diseases.
The roots of the plants growing in water still need oxygen at the correct levels to function properly. Not only the roots but also beneficial micro-organisms require oxygen to survive and thrive. However, the levels they require can differ from terrestrial plants. Although terrestrial plant roots seldom see ambient air concentrations (since the air must first diffuse through the porous structure of the soil), the levels they experience are much higher than the oxygen levels typically found in water.
It is very important to note that different gases dissolve in water at different rates and so will dissolve in water in different proportions to those in the air. For instance, CO2 readily dissolves in water but oxygen and nitrogen less readily.
Water will only hold a given amount of dissolved gases, which means that as more CO2 dissolves, other gases such as oxygen and nitrogen are driven out. Also, at higher temperatures or higher levels of salinity, a disproportionate amount of the less readily dissolved gases will come out of solution faster than the more readily dissolved gases such as CO2.
When should water be aerated and how?
There are two basic ways for air to get into water:
- it can dissolve into the water from the atmosphere under normal pressure, or
- it can be forced through the water artificially (oxygen diffusion).
While some fish and aquatic plants can extract enough oxygen to survive at concentrations at around 5 ppm, terrestrial plants cannot. Plants that are typically land based will need extra oxygen when grown in a water medium. However, a distinction needs to be made between whether a plant is being grown in water (deep-water culture or aquaponics), or just exposed to water on occasion (other methods).

When deep-water culture or aquaponics is used to grow terrestrial plants, the levels of oxygen dissolved must be raised beyond what would normally be absorbed by simply stirring the water. Depending on the water temperature and salinity levels, this could be a difficult task and the need for oxygen diffusion comes into play. There are some risks in this system especially if it is drawn from an environment that has been enriched with CO2.
Fluctuations in pH
This will cause fluctuations in pH, usually upwards as the CO2 combines with calcium. Additionally, less O2 will be dissolved because CO2 dissolves readily and displaces O2. It is important, then, to draw the air from an outside source and keep an eye on the pH. It will fluctuate and the more available the nutrient package is, the faster and more pronounced these fluctuations will be.
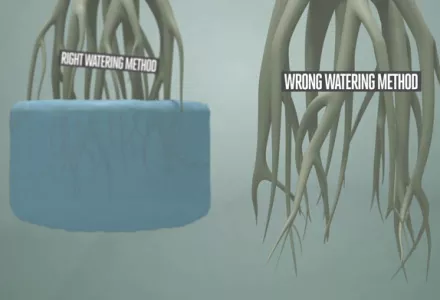
In all other systems in which water is applied and then the supply is stopped while it drains away, including those using clay pebbles, rockwool, sand, soil, peat, coco, or anything else where the roots do not actively sit in water all the time, aeration for oxygenation will not need to take place as intensively as for aquaponics.
The air that dissolves into the water naturally, with perhaps some stirring action for long-term tank preparations, will probably do just fine. This will help avoid stagnation, maintain levels of O2 appropriate for life, and keep the pH from swinging uncontrollably especially where the tank is located in a CO2-enriched atmosphere.
Additional oxygen for root health may well not needed because the action of the water draining will pull air into the pores of the medium and provide adequate levels of O2 in the film of water around the root surface. Most of the oxygen in the water will not be used since it will not stay long enough to be absorbed except from the solution on the root surface.
What’s more, roots that are not submerged the whole time are not the same as roots that live in water; there are differences in things like the thickness of the pericycle which controls the amount of water that moves into a plant. Submerging roots that have not developed under water for longer than twenty minutes will drown them.
In hybrid systems such as ebb and flow (flood and drain), the act of pumping the water up onto the table and then allowing it to flow back into the holding tank is sufficient to keep enough dissolved gases in the system. In environments with high levels of added CO2, greater than normal levels of CO2 can be dissolved into the water with similar results as air injection. However, this is nowhere near as rapid as the physical bubbling of air through the solution. It is necessary to keep a close eye on pH issues, and tanks need to be changed more frequently than would be required in non-supplemented rooms.
So, in systems that do not involve submerging the roots in water at all times, including systems that allow some water to be held in the medium against gravity but away from the root, it is best to limit air pumps and injection systems. This is because oxygen in these systems will mostly come from diffusion in the medium after irrigation: a simple system that stirs the water in the tank for a few minutes every hour or so will be enough to meet the needs of the system.
This could be as simple as a diverter pipe in the tank pump which directs a small amount of the pumped water back into the tank. Other devices could include mechanical stirring equipment like that used in the construction industry for paints and other mixing functions.

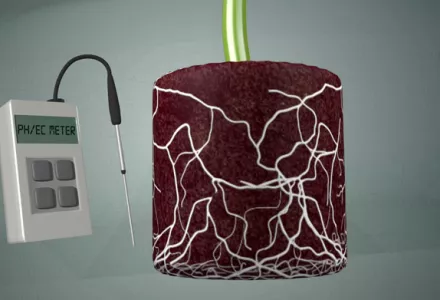
In truly hydroponic systems with an inert medium that holds little water, such as clay pebbles or Nutrient Film Technique (NFT), O2 concentrations at or above 40 ppm are required, or better still approaching 60 ppm, and achieving this may require more air to be dissolved and levels will have to be monitored closely. However, the air does not have to come from a diffuser. For aquaponics, because the volume of water will not be conducive to allowing appropriate O2 levels naturally, the solution used will have to be diffused with O2.
Regulation is critical
Air is certainly an important component in irrigation water, but regulation is critical to avoid upsetting the balance of the system. The real question is whether the extra work of diffusing air into the water is actually necessary, or even whether it is doing more harm than good.
The answer for you
The grower needs to be smart about what is needed, the results that can be expected, and what the true costs are. For any system except total root submergence, if stagnation is an issue for the grower even with the simpler techniques mentioned here, the answer may well be a smaller holding tank with more frequent renewals.