In this article we will look at pH and acidity and what this means for a plant and the growing environment. First of all, we’ll look at what acidity and pH really are.
What is acidity?
Acidity is essential for life on earth. Acidity often determines the characteristics, quality, absorbability and solubility of many substances. This is how enzymes, which are responsible for almost all biological processes in organisms, work, but only with the correct acidity[1]. A small fluctuation in the blood’s acidity is deadly.
What is pH?
The pH (pondus Hydrogenii) indicates a solution’s acidity or alkalinity[2]. The pH value usually varies between 0 and 14. A solution with a pH value between 0 to 7 is acid and one between 7 to 14 is alkaline. Vinegar and cola have a pH value of less than 3. Soda and soap have a pH value higher than 8. A pH value of 7 is considered neutral. Pure water at room temperature has a pH of 7. The pH of tap water is generally a little higher due to the presence of calcium.
Many natural environments such as our skin, plant substrates and nutrient mediums are mildly acidic and have a pH value of between 5 and 6.5. If we look at the things that people like we see that they are generally mildly acidic or neutral substances such as water. Plants also prefer mildly acidic substances. A pH value of around 5.5 occurs so often in nature that some plant experts regard this value as 'neutral'.
Why is acidity important?
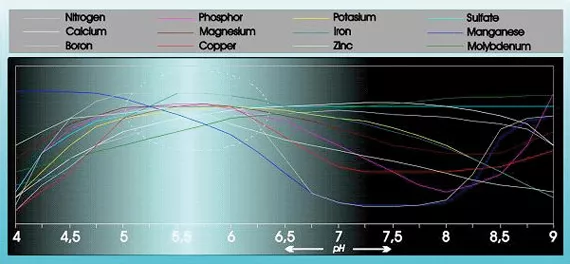
Acidity has a substantial influence on the absorbability and solubility of a number of food elements (see figure 1).
In addition acidity has considerable influence on the structure, breakdown of organic substances, and the micro life in the ground. The pH also influences the way in which food elements, heavy metals, and pesticides are flushed out of the ground.
A pH value that is too low or too high can be detrimental to your plants, so it is important to get it right. But how do you know when the pH is wrong? By experience! So to help you, we’ve set out some of the symptoms you might observe:
Symptoms of a pH that is too low (substrate is too acid):
- Most nutrients can be dissolved easily, which can cause an excess of manganese, aluminium and iron;
- Phosphorus, potassium, magnesium and molybdenum deficiencies can be caused by excessive rinsing;
- Magnesium deficiency, especially in cold substrates;
- The soil is generally poor;
- Soil life is inhibited.
Symptoms of a pH that is too high (substrate is too alkaline):
- Most nutrients dissolve less easily, causing calcium, iron and phosphate compounds to precipitate;
- Reduced absorption of manganese, phosphate, and iron in particular but also copper, zinc and boron. This will cause deficiencies, particularly in wet, cold growing mediums In sandy soils the breakdown of organic substances increases considerably if the pH is high.
What determines the pH?
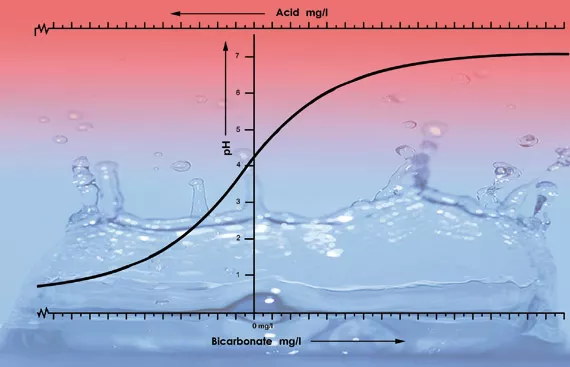
One of the most important factors determining the pH value in a solution or in the substrate is the buffering capacity. The buffering capacity in this instance means that there is a sort of balance present that continually restores itself. For example, if one puts a drop of acid into 1 litre of tap water that has a pH of 7 it will have little influence on the acidity. However, if one puts one drop of acid in 1 litre of demineralised water (battery water), the pH will immediately fall dramatically. This is because tap water contains bicarbonate while demineralised water doesn’t. Bicarbonate is the most important buffering substance for pH values between 5.5 and 7.5 in water[4].
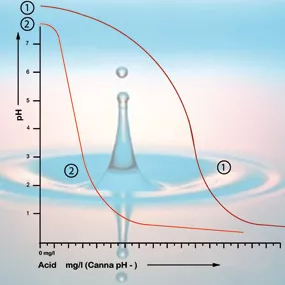
Bicarbonate binds itself to acid in the solution which releases carbon dioxide into the atmosphere. This is how the acid is neutralised and the changes in the acidity will only be minor so long as there is still bicarbonate present.
With a pH value of 5.3 all the bicarbonate has been used up and the solution has no more buffer. The pH is now unstable and it will change immediately if acid is added (see figure 2). The amount of acid that is needed to get a feeding solution to the correct acidity can therefore be calculated based on the bicarbonate content. The bicarbonate content of tap water is generally given by the water company in milligrams per litre[5].
The buffering capacity and the substrate’s acidity depend on its composition and freshness. The presence of organic material, calcium and bicarbonate generally determine the pH. Clay always contains calcium carbonate and has a relatively high pH value which is difficult to change, while peat and sandy soils are acid[6].
The plant itself also has great influence on the acidity. The roots will secrete either acid or alkaline substances depending on the crop’s stage of development, the food available, the differences in root temperature and light intensity. So you see why the pH of the root environment can constantly fluctuate. A sophisticated feeding balance during the different phases of development will keep the pH in the root environment within acceptable limits.
Micro life, CO2 levels, and algae growth can also have an effect on the acidity of the root environment and the nutrient tank[7].
Measuring the pH value
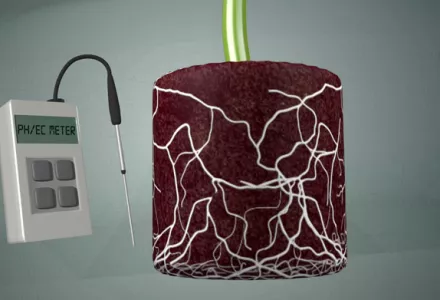
It is quite easy to measure the pH – you need some pH indicators such as litmus paper or a pH testing set. These are relatively cheap but are not always accurate and can sometimes deviate by 1 to 2 pH units. pH meters are generally more expensive and the accuracy depends on the type of meter and regular calibration with calibration fluid.
Taking samples

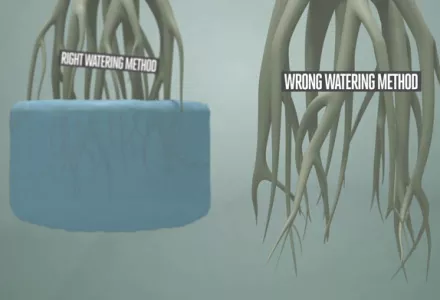
The pH of the water used to water the plants is important but the acidity around the roots is essential. So when you measure the pH it is very important to take the sample in the correct way to get good results. The sample has to represent the average acidity in the root environment.
It is easy to take samples and measure the pH in a recirculation system, simply measure the recirculated feeding solution.
In substrate systems without recirculation, feeding solution is drawn out of the substrate (rock wool, agrofoam) at a number of locations. Experts have been discussing the question of where to take the samples from for a year and a day. We recommend, just like a number of reputable laboratories do, taking samples from the places where the roots are which is under and around the drippers. Take small samples from as many places as possible. Always take all samples at the same time and preferably after the second drip-feeding during the light – daytime – cycle.

In soil, coco and peat substrates just take a small amount of substrate from several locations.
You can best measure the acidity of your sample using the '1:1.5 volume extract' method. You can easily do this yourself by making the growing medium so wet that the water runs through your fingers when it is kneaded and squeezed quite hard. Use a 250 ml measuring beaker for example. Fill the measuring beaker to 150 ml with demineralised water. Add growing medium until the volume is 250 ml (photo 3). Shake it well and let it stand for a few hours. Then filter it and measure the pH.

The correct pH values for every medium
When cultivating in substrate pH values of between 5.0 and 6.4 are fine for the root environment. There will not be any adverse effects if the values are a little higher or lower. Immediate adverse effects will only be seen with values lower than 4 and higher than 8. pH values lower than 4 often cause immediate damage to the roots. In addition, heavy metals, including manganese and iron are absorbed so well that they can poison the plant (necrosis). Values between 7 and 8 are not immediately harmful for the plant. Nutrients such as iron, phosphate, and manganese are less available then which will lead to deficiencies (chlorosis and development problems) in the long run.
Correcting the pH value
If the acidity in the root environment is between 5 and 6.4 then the pH of your growing environment is OK and you do not have to take any corrective measures. Try to avoid correcting the pH unless it is really necessary. It’s more likely to do harm than good; the plant likes its peace and quiet. It is more important to monitor how the acidity changes over a longer period. If the value falls below pH 5 or rises above pH 6.4 then it is advisable to gradually start making adjustments (see figure 2).
Correcting the acidity is most easily done by lowering the acidity of the feeding solution with nitric acid during the growing phase and phosphoric acid during the flowering phase or, as the case may be, to raise it with caustic potash, potassium bicarbonate or soda[8] and CANNA RHIZOTONIC. Ensure that the pH in the solution that is used does not fall too far below 5.0. When growing in rock wool the fibres will be harmed causing a lot of alkaline material to be released at very low pH values. In addition, the pH is more difficult to control due to the absence of bicarbonate.
A high pH in the root environment can also be caused by bicarbonate that has built up. To remedy this maintain 20% drainage or rinse through with a more acid solution.
It is useful to note the pH measurements from both the solution added and the feeding solution in the substrate. You will get a good idea of the progression of the pH and the effect of the measures taken.
- Protein splitting enzymes need an acidic environment (gastric juices) and carbohydrate splitting enzymes need an alkaline environment (saliva).
- The acidity of a solution is determined by the ratio of hydrogen ions (= acid) and hydroxide ions (= alkaline).
- Shortages can occur because the plant has to secrete protons to be able to absorb these molecules. A growing medium with a low pH already has a very large quantity of protons. These elements also get rinsed out because the protons repel the molecules from the medium in the substrate.
- Bicarbonate is the substance that, when combined with calcium, is responsible for scale. In combination with sodium, bicarbonate is used in medicaments to counter excess gastric acid (Alka-seltzer).
- Some laboratories also work with the bicarbonate hardness. In order to translate this to mg/l bicarbonate you must multiply the bicarbonate hardness by 21.8.
For example: the bicarbonate hardness is 11, then 1 litre of water contains (11 x 21.8=) 240 mg/litre bicarbonate. -
Sandy ground: Grass land pH 4.6 … 5.2 Building land pH 5.0 … 5.6 Clay: Sea Clay pH 6.0 … 7.2 River Clay pH 6.2 … 6.4 Peat: Unprocessed pH 4.0 - If there is significant algae growth then the pH will increase because carbon dioxide will be removed from the solution. Bacteria can transform certain forms of nitrogen so that they have an acidifying effect. Large amounts of CO2 in the air generate more carbon dioxide in the feeding solution and vice versa.
- Only use soda in small quantities, because it contains sodium, and plants only need a very small quantity of sodium. Remember, high concentrations of sodium will damage the plant.